Case Management &Resistance Bill Brieger | 22 Oct 2017 10:46 am
The Need to Prevent the Spread of Malaria Drug Resistance to Africa
Chike Nwangwu is a Monitoring and Evaluation Specialist who is currently working on his Doctor of Public Health (DrPH) degree at the Johns Hopkins Bloomberg School of Public Health. Here he presents an overview of the threat of parasite resistance to first-line antimalarial drugs and the need to prevent the spread of this problem in Africa which beard the greatest burden of the global malaria problem.
Malaria, remains one of the most pervasive and most malicious parasitic infections worldwide. Malaria is caused by Plasmodium parasites when they enter the human body. There are currently five known plasmodium species that cause malaria in humans- P. falciparum and P. vivax are the most prevalent globally. These parasites are transmitted through the bites of infected female anopheles mosquitoes “malaria-vectors” which perpetuate the spread of the parasite from human-human or from host- human.
Globally, according to the WHO, an estimated 212 million cases of malaria and 429 000 malaria related deaths occurred in 2015.[1] The global share of malaria is spread disproportionately across regions; Over 90% of global malaria cases and deaths occurred in the African region, with over 70% of the global burden in one sub region-Sub Saharan Africa. In areas with high transmission of malaria, children under 5 are at the highest risk to infection and death; more than two thirds of all malaria deaths occur in this age group.[2]
 Although malaria remains a global concern, malaria is preventable and curable. Increased efforts in malaria prevention and treatment within the past two decades has led to revolutionary success- 6.8 million lives have been saved globally and malaria mortality cut by 45% since 2001.[3] Globally, within a five-year interval (2010-2015) new malaria transmission and mortality in children under 5 years of age fell by 21% and 29% respectively. This has been the one the greatest public health successes in recent years [4]
Although malaria remains a global concern, malaria is preventable and curable. Increased efforts in malaria prevention and treatment within the past two decades has led to revolutionary success- 6.8 million lives have been saved globally and malaria mortality cut by 45% since 2001.[3] Globally, within a five-year interval (2010-2015) new malaria transmission and mortality in children under 5 years of age fell by 21% and 29% respectively. This has been the one the greatest public health successes in recent years [4]
The improvement of malaria indices aligns with intensification of efforts, through funding, research, innovation pushing the scale up of key malaria interventions in the malaria prevention- diagnostic- treatment cascade. For example, in Sub-Saharan Africa where malaria is most prevalent, there has been a recorded 48% increment in Insecticide Treated Net (ITN) usage since 2005, 15% rise in chemoprevention in pregnant women and within the same time frame diagnostic testing increased from 40% of suspected malaria cases to 76%.[5]
Treatment/ Emergence of insecticide and drug resistance
Malaria treatment plays a key role in controlling its transmission. First, prompt and effective treatment of malaria prevents progression to severe disease and limits the development of gametocytes, thus blocking transmission of parasites from humans to mosquitoes.[6] Drugs can also be used to prevent malaria in endemic populations, including various strategies of chemoprophylaxis, intermittent preventive therapy, and mass drug administration can be effective.[7] Like other interventions, availability and use of antimalarial has been a success. However, this has also come with some challenges. The emergence of resistance, particularly in P. falciparum and P. vivax to antimalarial-quinine and sulfadoxine-pyrimethamine, has been a major contributor to reported resurgences of malaria in the last three decades.[8]
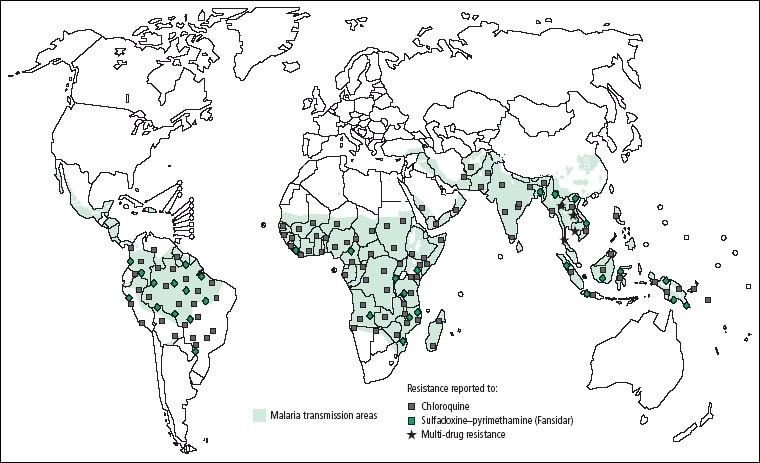
Distribution of reported resistance to antimalarials. As at 2005, antimalarial resistance was is established in 81 of the 92 countries where the disease was endemic (WHO, 2004)
Falciparum resistance first developed in some areas in Southeast Asia, Oceania, and South America before the 70’s eventually the parasite became resistant to other drugs (sulfadoxine/ pyrimethamine, mefloquine, halofantrine, and quinine. Drug-resistant P. vivax was first identified in 1989 in one region and later spread to other regions of the world.[9] As at 2005, antimalaria resistance to chloroquine and sulfadoxine-pyrimethamine was established in 81 of the 92 countries where the disease was endemic. (Figure 1) In 2005, alongside acetaminophen, antimalarial were among the most commonly abused medications in the African region, with the majority of the population having detectable amounts of chloroquine in the blood.[10]
Antimalarial drug resistance is the decrease in viability of an antimalarial to cure an infection. Parasite resistance results in a delayed or partial clearance of parasites from the blood when a person is being treated with an antimalarial.[11] Antimalaria resistance occurs as the byproduct of at least one mutation in the genome of the parasite, giving an advantageous capacity to evade the impacts of the drug. Within the human host, drug resistance develops gradually. First, a modest number of drug-resistant parasites survive exposure to the drug whilst the drug-sensitive parasites are eliminated. In the absence of additional drugs and competition from drug-sensitive pathogens, the drug-resistant parasites proliferate and their populace develops. This new population is therefore resistant to additional malaria medications of the same type.
Following the discovery of resistance to quinine and sulfadoxine-pyrimethamine, the development of resistance was initially forestalled by the utilization of a new class of malaria drugs – Artemisinin-derivative combinations. These ACTs (Artemisinin Combination Therapy) work by combining artemisinin and an active partner drug with different mechanisms of action. The WHO, with guidance from extensive drug efficacy tests and research, recommends the use of 5 types of ACTs for treatment of uncomplicated malaria caused by the P. falciparum parasite. By 2014, ACTs have been adopted as first-line treatment policy in 81 countries.[12]
Although ACT use has been a breakthrough in malaria treatment, development of resistance to de novo ACTs poses one of the greatest threats to malaria control efforts. P. falciparum resistance to artemisinin has been detected in five countries of the Greater Mekong sub-region (Lao, Myanmar, Thailand, Cambodia, and Vietnam). To date P. vivax resistance to an ACT has not been detected.
Artemisinin resistance is currently defined within the confines of delayed parasite clearance; it represents partial/relative resistance-i.e. most patients who have delayed parasite clearance do not necessarily have treatment failure. Following treatment with an ACT, infections are still cleared, as long as the partner drug remains effective. Various factors are believed to contribute to the development and spread of resistance to artemisinin; use of oral artesunate monotherapies (oAMT) inclusive.
 A global response has been mounted to curtail the spread of ACT resistance to other regions, especially to regions like Sub-Saharan Africa. Research on the mechanisms of drug resistance has steered efforts in the direction, recently the identification of the PfKelch13 (K13) mutations has allowed for a more refined definition of artemisinin resistance that includes information on the genotype. [13] In addition, stricter policies have been developed for malaria control; Therapeutic efficacy studies (TES) are conducted and used as the main reference from which national malaria control programmes determine their national treatment policy.[14] These studies help to ensure the efficacy of treatments with recommendations to ensure that these medicines are monitored through surveillance at least once every 24 months at established sentinel sites and in regions with emerging resistance, the creation of additional sentinel surveillance sites. [15]
A global response has been mounted to curtail the spread of ACT resistance to other regions, especially to regions like Sub-Saharan Africa. Research on the mechanisms of drug resistance has steered efforts in the direction, recently the identification of the PfKelch13 (K13) mutations has allowed for a more refined definition of artemisinin resistance that includes information on the genotype. [13] In addition, stricter policies have been developed for malaria control; Therapeutic efficacy studies (TES) are conducted and used as the main reference from which national malaria control programmes determine their national treatment policy.[14] These studies help to ensure the efficacy of treatments with recommendations to ensure that these medicines are monitored through surveillance at least once every 24 months at established sentinel sites and in regions with emerging resistance, the creation of additional sentinel surveillance sites. [15]
Preventing and containing antimalarial drug resistance- Recommendations to countries
As research is being done to fully understand the mechanisms of antimalarial resistance; basic recommendations to limit its spread have been disseminated.[16] First, the production and use of oral artemisinin-based monotherapy should be halted and access to the use of quality-assured ACTs for the treatment of falciparum malaria should be ensured. In countries where antimalarial treatments remain fully efficacious; correct medicine use must be promoted, with weight placed on encouraging diagnostic testing, quality-assured treatment, and good patient adherence to the treatment. Lastly, to reduce the burden of the disease, and prevent the spread of resistance, in regions where there is still high transmission, intensification of malaria control efforts is key, rapid elimination of falciparum malaria would accelerate efforts.
- [1] World Malaria Report 2016, World Health Organization, Geneva, 2016
- [2] ibid
- [3] CDC, Malaria Fast Facts 2017
- [4] ibid
- [5] World Malaria Report, 2016
- [6] Gosling RD, Okell L, Mosha J, Chandramohan D. The role of antimalarial treatment in the elimination of malaria. Clin Microbiol Infect. 2011;17:1617–1623.
- [7] Greenwood B. Anti-malarial drugs and the prevention of malaria in the population of malaria endemic areas. Malar J. 2010;9
- [8] White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092
- [9] CDC, Malaria Fast Facts, 2017
- [10] White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092.
- [11] Peter B. Bloland, Drug resistance in malaria, World Health Organization, 2001
- [12] World Malaria Report, 2016, World Health Organization, Geneva,2016
- [13] Artemisinin and artemisinin-based combination therapy resistance April 2017,World Health Organization, 2017
- [14] Responding to antimalarial drug resistance, World Health Organization, 2017: http://www.who.int/malaria/areas/drug_resistance/overview/en/
- [15] ibid
- [16] Ibid
on 22 Oct 2017 at 8:23 pm 1.The Need to Prevent the Spread of Malaria Drug Resistance to Africa said …
[…] See the original post here: The Need to Prevent the Spread of Malaria Drug Resistance to Africa […]